New Hope for Babies: Screenings for Spinal Muscular Atrophy
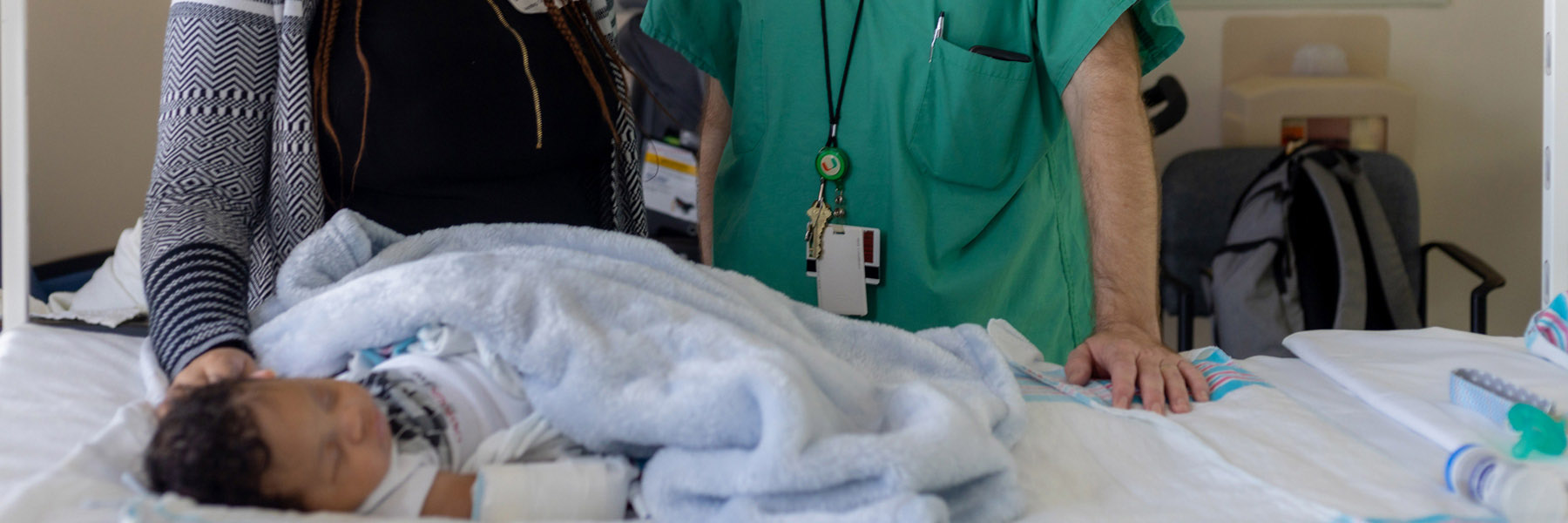
All babies born in the U.S. receive a newborn screening to detect genetic anomalies and potentially guide treatment.
These routine screenings usually come back clear, but Roxanne Brown knew it was bad news the instant she heard the pediatrician’s voice. “The doctor only calls when something is wrong,” she said.
In Jasye Bowen’s case, the test came back positive for a potentially fatal condition called spinal muscular atrophy (SMA).
SMA is a genetic disorder that causes all of the muscles in a person’s body to lose strength over time.
Brown researched the condition online, and that only made matters worse. Jasye had the most severe form of SMA, type 1. In many cases, children die before their second birthday.
But there was also good news. Jasye was diagnosed because of a recent change in Florida’s Newborn Screening Program.
Since late April, all newborn babies in the state have been screened for spinal muscular atrophy.
Born on May 1, Jasye was the first child with SMA identified by the program. Even better, the disease could be treated with a new gene therapy.
“This was the first condition where we had a newborn screening for a gene therapy,” said Deborah Barbouth, M.D., a geneticist with the University of Miami Miller School of Medicine’s world-renowned John P. Hussman Institute for Human Genomics, associate professor of clinical human genetics and medical director of newborn screening for South Florida. “The other conditions we screen for are mostly treated with diet, vitamins or, in some cases, enzyme replacement therapies.”
Gene therapy: correcting the problem at the source
Jasye’s family was immediately referred to the University of Miami Health System, which offers South Florida’s only designated treatment center for SMA.
Jasye received is a drug called Zolgensma, which received FDA approval in 2019 and is the second gene therapy drug to be approved. The therapy works by adding a normal gene (SMN1) to replace the defective gene.
“Now that there is a gene therapy for this disease, if we can diagnose it before the child develops symptoms, we can either prevent it entirely or reduce the deleterious effects,” said Roberto Lopez Alberola, M.D., a UHealth pediatric neurologist treating Jasye.
We can reduce the ultimate outcome of that gene and change the trajectory of the disease to minimize its effects.
Dr. Roberto Lopez Alberola
SMA can move fast.
Within weeks of birth, Jasye’s neck and chest muscles were already deteriorating. In early June, he became the first child in Florida to benefit from this gene therapy, receiving his one-time infusion at Holtz Children’s Hospital in Miami. Soon after treatment, his parents noticed the change — Jasye could push out his chest and turn his head.
“It’s fascinating how this thing started to work,” Brown said. “I’m thinking that he will meet all of his milestones.”
Though this treatment is an important step forward, it may not be a cure. “There have been some cases reported where children do reach normal development with this form of gene therapy,” Dr. Lopez Alberola said, “but even in the ones who don’t necessarily achieve normal development, it is a significant improvement from where they would have been.”
A significant improvement
Still, for physicians and parents who have watched children with SMA die for years, the success of this gene therapy — and hopefully more to follow — is a tremendous breakthrough.
“These children used to come in with the most difficult genetic condition,” Dr. Barbouth said. “I would have to tell parents, ‘Even though your child looks fine, they are probably going to die within a year.’ It’s the most horrendous thing you can ever say. And now, knowing I will see them walk and talk and run, I’m crying from happiness.”
The Miller School’s Hussman Institute uses the very latest in cutting-edge technologies to identify genes involved in human diseases. Similar to Jayse’s case, Hussman experts work closely with UHealth physicians across multiple disciplines to advance disease diagnoses, and intervene and prevent illness.
With 25 years of experience in neurogenetics, Dr. Lopez Alberola said that the availability of gene therapy to treat patients was the vision of the federally funded Human Genome Project.
“Years ago, the science and medical community set out to establish innovative therapies that would correct diseases at their source,” he said. “With the availability of gene therapy, we are now reaping the fruit of that initiative.”
Originally written by Josh Baxt for Inventum. Photos courtesy of Holtz Children’s Hospital.
Tags: Dr. Deborah Barbouth, Dr. Roberto Lopez Alberola, HIHG, newborn screening, spinal muscular atrophy
