Not Every COVID-19 Test is the Same
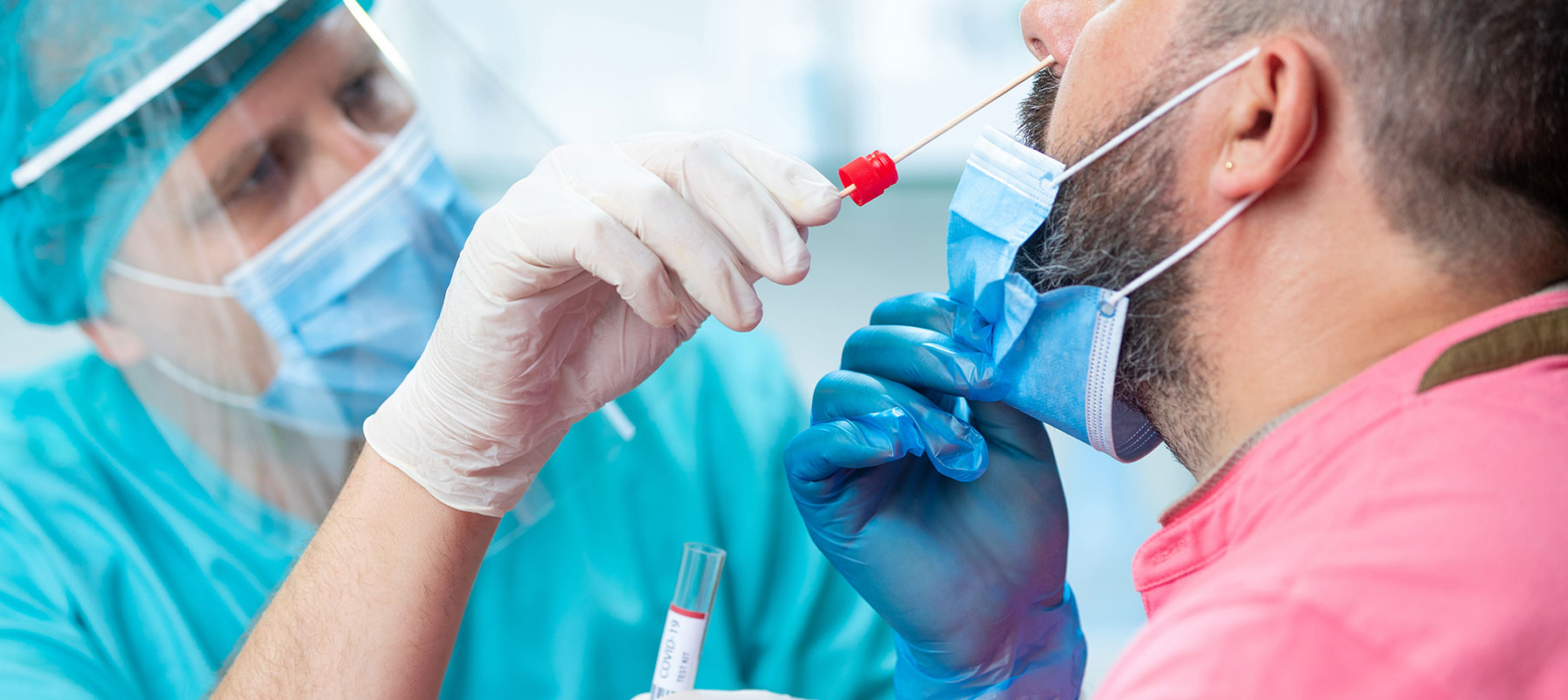
The pandemic has focused a spotlight on the importance of accurate medical tests, especially in light of recent increases in coronavirus cases.
“The important thing to remember,” says Merce Jorda, M.D., who serves as chair of pathology and laboratory medicine at the University of Miami Health System, “is that there are multiple types of tests with different collecting methods, which vary in accuracy.”
At last count, the U.S. Food and Drug Administration (FDA) has awarded emergency-use authorization to more than 200 different tests meant to detect SARS-CoV-2. And there are more in the pipeline. Testing, as Dr. Jorda explains, is essential to containing the virus because it helps public health officials spot – and contact trace – other infected patients, even those who are asymptomatic.
There are three different kinds of COVID-19 testing:
- molecular real-time polymerase chain reaction (RT-PCR) tests, which detect the virus’ genetic material
- antigen tests that detect specific proteins on the surface of the virus
- serology (antibody) tests, which detect antibodies to SARS-CoV-2 in blood
The first two types are diagnostic tests, while serology is considered adequate for surveillance protocols. In other words, a diagnostic test detects an active coronavirus infection while a serology test identifies a past infection.
“While there is no perfect test, the RT-PCR is considered the gold standard because of its accuracy,” Dr. Jorda says.
The RT-PCR test can detect tiny parts of the virus’s RNA, its genetic material.
This is collected using a swab inserted in the nasal cavity (deep or midway) or by obtaining saliva. In the lab, the viral RNA is converted to DNA through a process called “reverse transcription.” This allows the DNA to be copied or amplified, which is a crucial part of the RT–PCR process. If the viral DNA is detected, the patient is considered positive for SARS-CoV-2, the COVID-19 virus.
Though false-positive results are rare, RT-PCR has missed some infections, mostly due to the infection stage or erroneous sampling. Furthermore, different platforms have different levels of viral detection. People also have been known to continue testing positive even when they’re not actively sick. Physicians believe this happens because viral genetic material can hang out in the body for some time.
Another drawback of the RT- PCR is that testing supplies may be scarce, and it can take several days to process in a lab. At UHealth, under Dr. Jorda’s direction, Dr. Yi Zhou leads an in-house COVID-19 laboratory for employees, students, and patients. The in-house nature of the COVID-19 testing allows for a better rapid turnaround time of the sample results.
“Having an in-house laboratory helps tremendously,” says Dr. Jorda. “In a commercial lab, you have to get in line with everyone else.”
Some find the deep nasal collection method uncomfortable. So, other less-invasive collection methods have been developed. In August 2020, the FDA gave the emergency-use designation to a saliva-based test named SalivaDirect. Though this is not the first saliva test approved for COVID-19 testing, it received a lot of attention since this method shortens the testing time. It is considered as accurate as the nasal RT-PCR test.
The Antigen test, like the RT-PCR, requires a nasal swab.
Instead of detecting the RNA of the SARS-CoV-2 virus, the antigen test searches for proteins on the virus’s surface. Since it does not require as many testing reagents and laboratory staffing, it is cheaper and faster than RT-PCR.
However, quick turnaround time translates into less accuracy. False negatives are much more common than with RT-PCR. Subsequently, the FDA recommends an RT-PCR test if a patient’s antigen test is negative but displays symptoms of COVID-19.
The White House press secretary’s case may be an example of this. She released a statement on Twitter that she had tested negative for several days before finally testing positive, using the antigen test. While the White House has reportedly used a rapid antigen test to screen visitors, the RT- PCR tests still need to be used as a backup, as advised by the FDA.
However, if antigen testing is done frequently, the positive cases will most certainly be picked up on subsequent samples. False-positive results are common with the antigen test because it detects proteins that appear like those of SARS-CoV-2. That said, antigen tests are helpful when speed is of the essence, and when they are performed repeatedly.
The antibody test, unlike the RT-PCR or antigen tests, measures if a person has been infected by the coronavirus in the past.
Using a blood sample, the test looks for antibodies against SARS-CoV-2, which are the proteins that our bodies make after an infection to protect us from future threats. Right now, antibody tests provide scientists with an idea of the virus’s reach in a community. Still, a positive test does not mean that the tested individual is immune to SARS-CoV-2, and potential reinfection can occur. Therefore, universal precautions measures should remain in place.
Additional collection methods and testing protocols are currently being explored globally.
“The search for an inexpensive, fast, simple, and accurate test is on and will be forthcoming in the near future,” Dr. Jorda says, adding she wouldn’t be surprised if we soon have a test with those characteristics.
However, she says: “In the meantime, we must all continue with the precautions of using masks and social distancing.”
Ana Veciana-Suarez, Guest Columnist

Ana is a regular contributor to the University of Miami Health System. She is a renowned journalist and author who has worked at The Miami Herald, The Miami News, and The Palm Beach Post.
Tags: COVID testing, COVID-19, Dr. Merce Jorda
