Boy with Inherited Blindness Receives FDA-Approved Gene Therapy
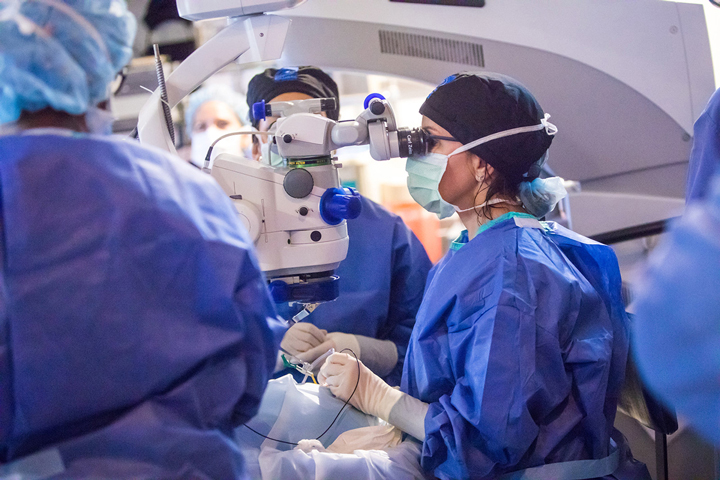
On March 22, Creed Pettit, a 9-year-old legally blind boy from Mount Dora, FL, became the first patient to undergo a landmark gene therapy surgery at Bascom Palmer Eye Institute, part of the University of Miami Health System.
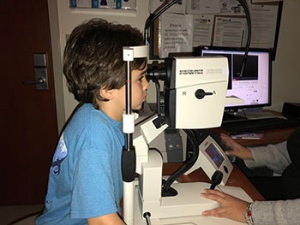
Creed was diagnosed at about age 2 with Leber congenital amaurosis, a blinding genetic disease caused by an RPE65 genetic mutation. His family, especially his mom Sarah St. Pierre Pettit, has been on a tireless journey to find a treatment for Creed’s condition, which impedes his vision in low light. While he’s able to draw, play the piano and enjoy other activities during daylight, at night, she said, Creed experiences complete darkness.
Most affected patients have decreased vision before the age of 10 from retinal degeneration. Luxterna, a product developed by Spark Therapeutics, is the first ocular gene therapy drug approved by the Food and Drug Administration (FDA). The drug, which was approved in December 2017, is a genetically altered viral vector that makes the photoreceptor cells on the retina produce a protein that people with the RPE65 genetic mutation lack.
Dr. Audina Berrocal, a pediatric retina surgeon at Bascom Palmer, performed the hour-long surgery on Creed’s right eye, using a hair-thin needle to inject the drug directly under his retina. Creed is scheduled to undergo gene therapy on his left eye on March 28.
“Gene therapy is the beginning of how we treat inherited eye disorders,” said Berrocal. “The treatment combines technology, subspecialty care, and advance science.”
Dr. Byron Lam, a Bascom Palmer ophthalmologist, research scientist and inherited gene expert who diagnosed Creed at the hospital years ago, said:
The FDA’s approval of the drug sets the precedent that gene therapy is a viable option to restore sight for people with inherited eye diseases.
Lam, a professor of ophthalmology, also noted the importance of genetic testing so that genetic eye disorders are “accurately categorized” in the event that a gene therapy is available.
Bascom Palmer has an extensive gene therapy program with ophthalmologists, surgeons, genetic counselors and research scientists studying various forms of inherited eye diseases.
Tags: Bascom Palmer Eye Institute, child blindness, Dr. Audina Berrocal, Dr. Byron Lam, FDA approval, gene therapy, Miami eye care
